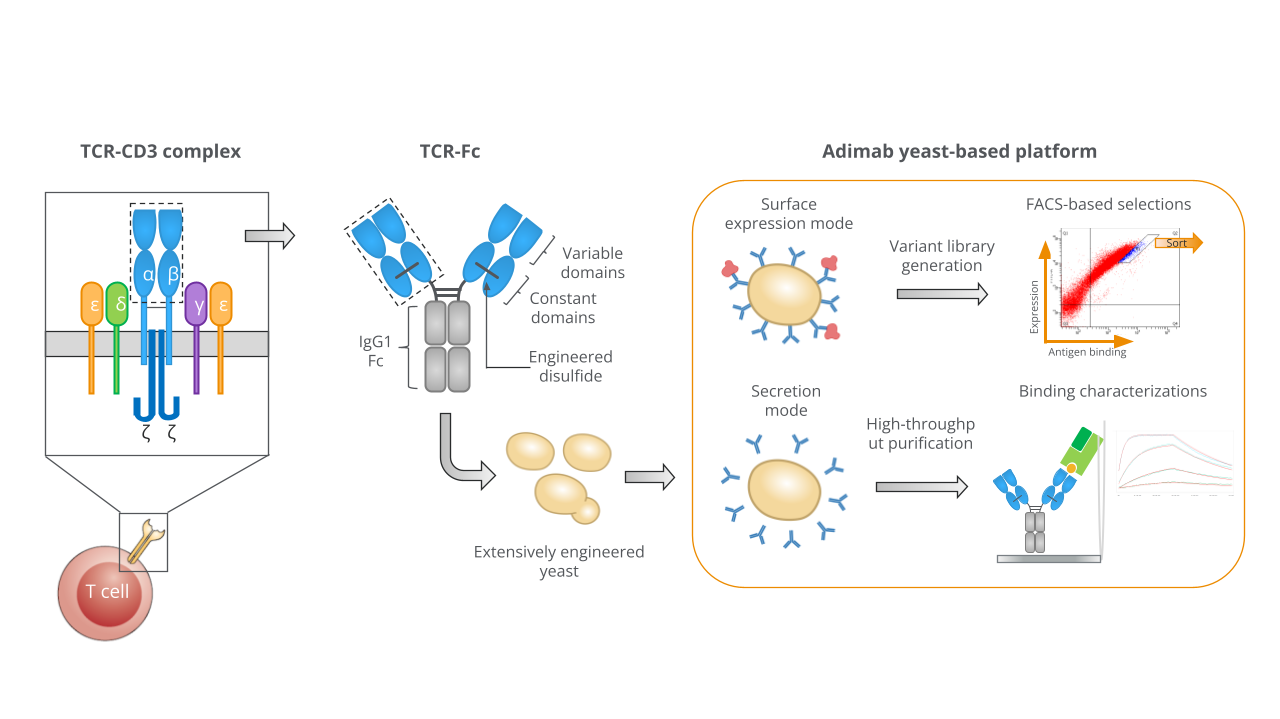
Soluble or surface TCR formats for fast selection.
All-CDR and DMS enable massive affinity gains.
TCR-Fc affinities match published references.
Optimized TCRs boost T cell activity >1000×.
Adimab’s yeast-based protein engineering platform supports expression of TCR-Fc molecules in both soluble and surface-presented modes. This dual capability allows rapid purification, screening, and kinetic analysis, while engineered constant regions and stabilizing disulfides enhance solubility and stability, accelerating workflows in T cell receptor engineering for therapeutic development.
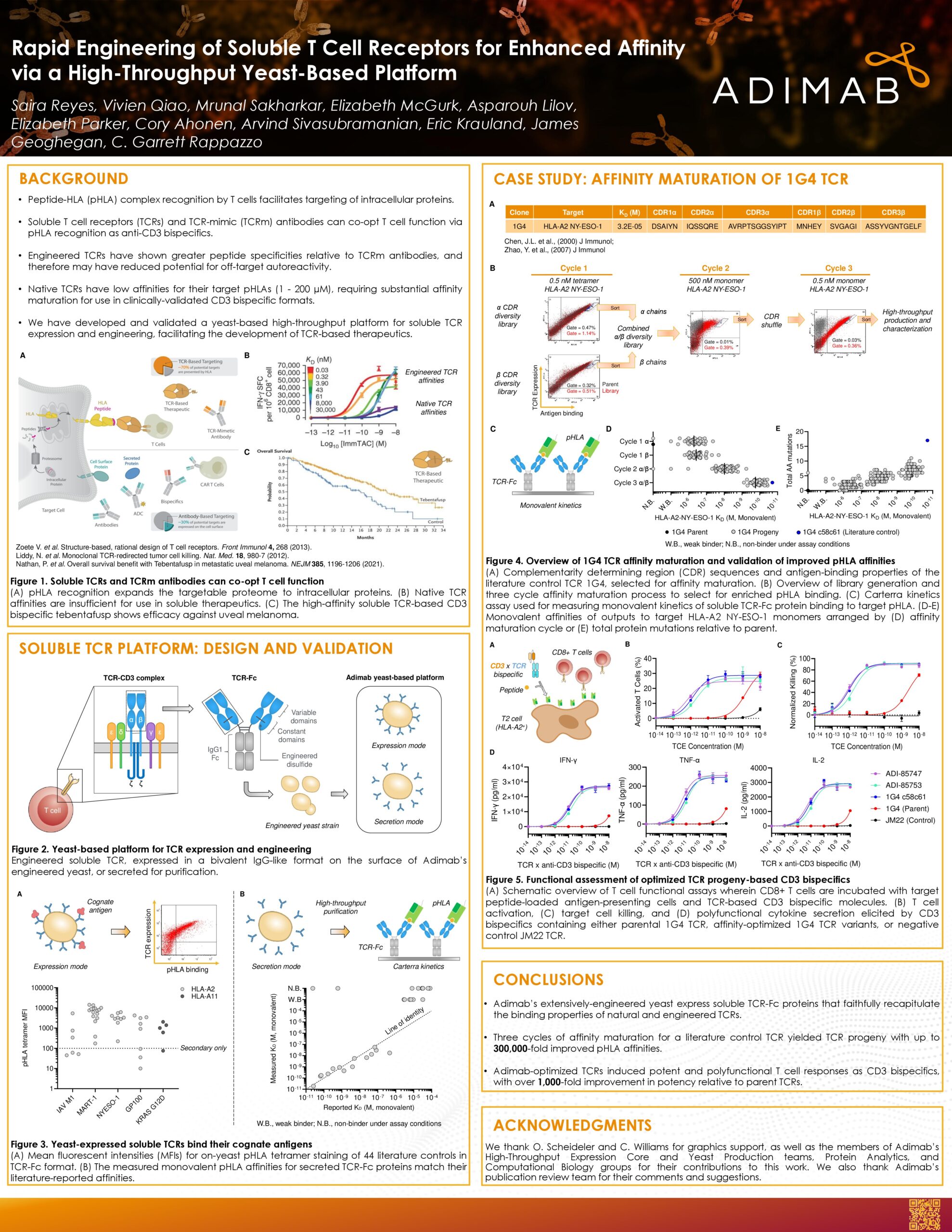
Using all-CDR diversification and deep mutational scanning, Adimab delivers dramatic affinity gains in TCR development. Optimized variants have achieved up to 300,000-fold tighter binding with fewer mutations than benchmark systems. These methods balance specificity and stability, advancing the potential of modern protein engineering platforms.
The yeast-based system has been validated across dozens of receptors and peptide–HLA complexes, consistently reproducing reported binding behaviors. This reproducibility highlights the reliability of our protein engineering platform in supporting diverse therapeutic projects, from early TCR discovery to advanced T cell engineering pipelines targeting intracellular antigens.
Engineered TCRs can be directly integrated into bispecific or other therapeutic constructs, demonstrating enhanced functional activity while preserving peptide selectivity. By combining high affinity, solubility, and specificity, Adimab’s protein engineering platform enables our partners to generate optimized receptors that translate into potent, clinically relevant molecules, including TCRs and other engineered proteins.

Our proprietary yeast-based protein engineering platform expresses soluble TCR-Fc molecules that align with published affinities across 44 diverse TCRs.
This system delivers reproducible binding behavior across peptide–HLA complexes and germline combinations, providing a reliable foundation for therapeutic development. The platform also produces engineered proteins that have modulated specificity and/or affinity to their natural partners.

With all-CDR diversification, TCRs engineered using our platform achieve up to 300,000-fold tighter binding in the 1G4 lineage, which recognizes the cancer antigen NY-ESO-1, and ~10,000-fold in MEL5, which targets the melanoma antigen MART-1.
These affinity gains are achieved with fewer amino acid substitutions than phage-derived controls, underscoring the efficiency of our high-throughput protein engineering workflow.
Optimized TCRs incorporated into CD3 bispecifics drive more than 1,000-fold stronger CD8+ T cell activation, cytokine release, and target-cell killing compared to parental clones.
The engineered molecules demonstrate therapeutic impact in formats designed to redirect immune responses.
Alanine scanning shows that affinity maturation using Adimab’s protein engineering platform preserves, and often enhances, peptide specificity.
Optimized 1G4 variants display sharper selectivity than affinity-matched controls, proving that high affinity and precise targeting can be achieved together in engineered receptors. Protein specificity can also be modulated using similar approaches.
Data-driven yeast platform delivers potent, peptide-specific TCRs ready for therapeutic development.

Engineered anti-CD3 HCAbs enable potent, tunable, and developable binders for next-generation TCEs.
Yeast-based engineering yields high-affinity soluble TCRs, enabling potent, precise T cell therapeutics.
Access high-quality synthetic and immune libraries delivering therapeutic antibodies for the clinic.
Transform leads into high-affinity, developable candidates through guided engineering and real-time screening.
Engineer multispecifics, including T-cell engagers, of diverse formats for function, manufacturability, and scalability.
Learn how our integrated capabilities in discovery and engineering help advance your programs to the clinic.