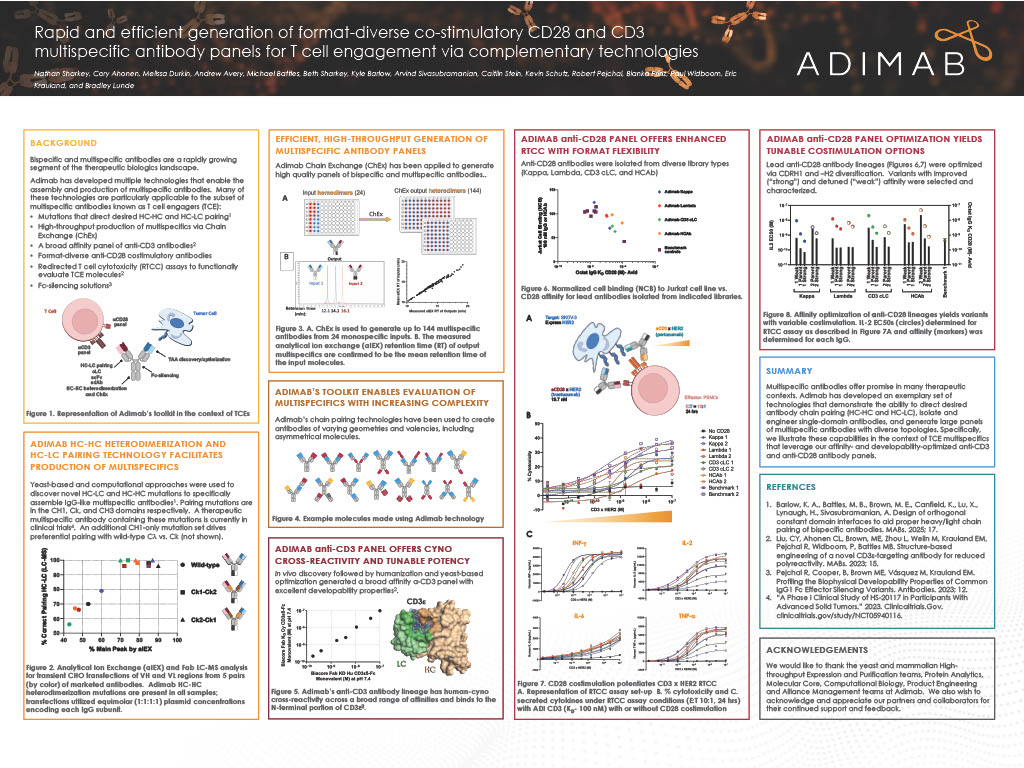
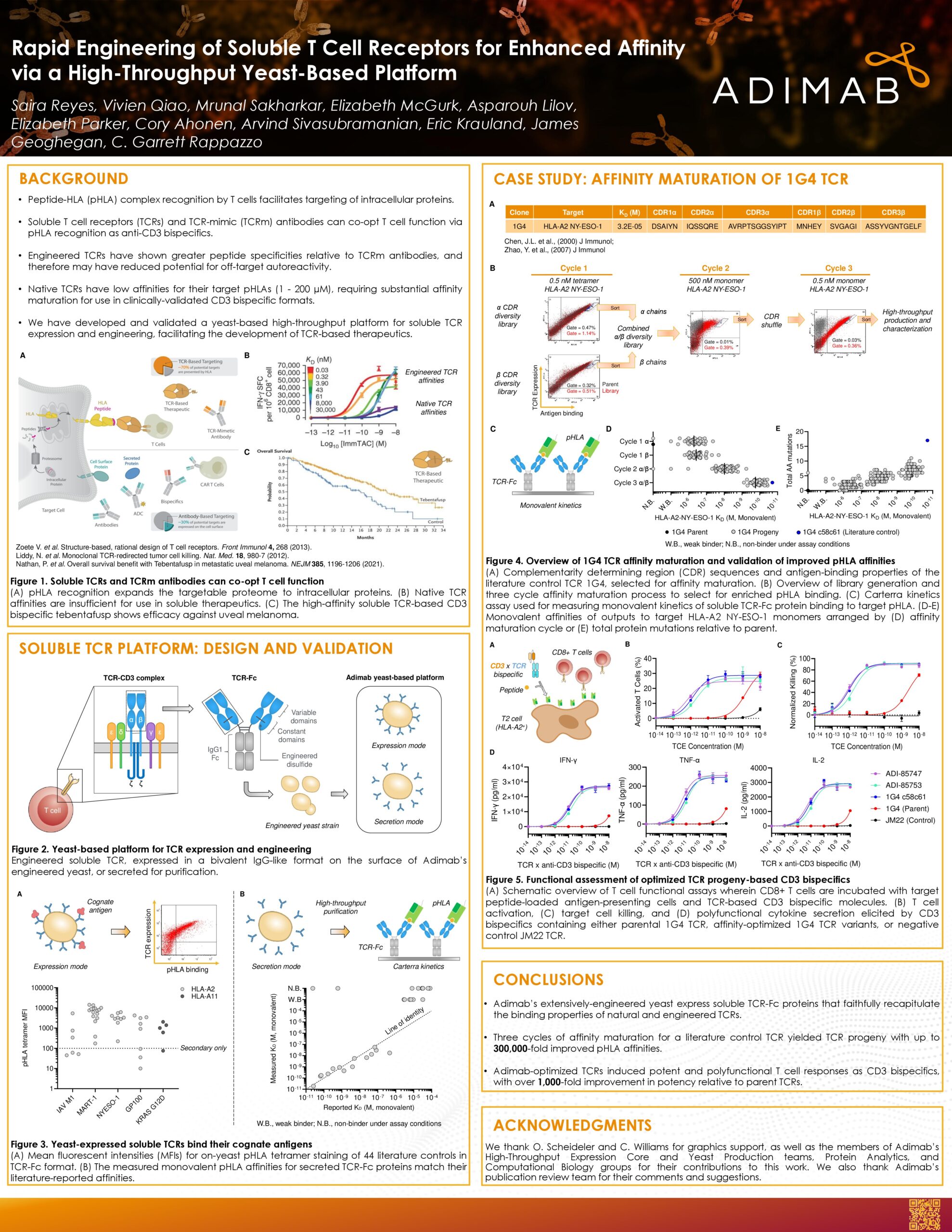
Biopharma programs often stall between hit discovery and the clinic due to specificity, developability, and timeline risks. This poster summarizes real-world case studies and platform metrics to show how Adimab’s end-to-end yeast-based workflow (diverse libraries, high-throughput production with analytics, and iterative optimization) converts early binders into clinical molecules with high specificity and manufacturability.
What the platform delivers
- Primary discovery typically yields ~100 diverse IgGs in ~3.5 months, from which 6–8 nominees can be advanced for optimization; thousands of unique IgGs can be produced per week (20 µg to 1 mg) for rapid characterization.
- More than 130 partners have leveraged the platform for 600+ therapeutic programs, contributing to over 80 clinical advancements and five commercial products (Tyvyt, Pemgarda, Sintbilo, Fucaso, Unloxcyt).
Case study 1: TTX-080 (HLA-G, Tizona Therapeutics)
- Adimab’s primary discovery efforts delivered 70 HLA-G binders: 34 were HLA-G–specific, and 8 passed LabScreen specificity testing against 94 HLAs, from which 5 were nominated for optimization.
- CDR-wide diversification and selections for specificity and affinity produced progeny with improved monovalent KD, retained developability (HIC/PSR), and enhanced functional blockade of ILT2-mediated suppression.
Case study 2: Solnerstotug (VISTA, Sensei Biotherapeutics)
- pH-pressure selections enriched binders with strong affinity at pH 6.0 and minimal binding at pH 7.4: 81 IgGs were delivered and 36 were strongly pH-selective, with good developability profiles across the set.
- Optimization improved both affinity and pH dependency; solnerstotug blocks PSGL-1-VISTA interaction and shows efficacy in tumor models, with ongoing Phase I/II evaluation.
Why it matters
Together, these data demonstrate Adimab’s discovery platform provides a reproducible route from antigen to clinic: specificity filtering at scale, quantitatively improved affinity and pH selectivity where needed, and consistent developability profiles, thereby reducing attrition and compressing timelines while supporting clinical progression.
View poster