Expanding access to intracellular targets
T cell receptor (TCR) therapeutics extend the reach of protein-based drugs into the intracellular proteome by targeting peptide–HLA (pHLA) complexes. However, native TCRs typically bind their targets with low affinity and are difficult to express as soluble, stable molecules, making affinity engineering both essential and challenging. Our yeast-based discovery platform provides an efficient, data-driven solution for generating high-affinity, highly developable, and peptide-specific TCRs at scale.
A unified system for display and secretion
Our platform enables expression of disulfide-stabilized TCR-Fc molecules on yeast in an IgG-like bivalent format. The same construct can be used for both surface presentation and secretion, allowing FACS-based selections and rapid biophysical characterization without reformatting or subcloning. This integrated approach accelerates iterative optimization, shortens timelines, and yields TCRs that are immediately suitable for therapeutic evaluation.
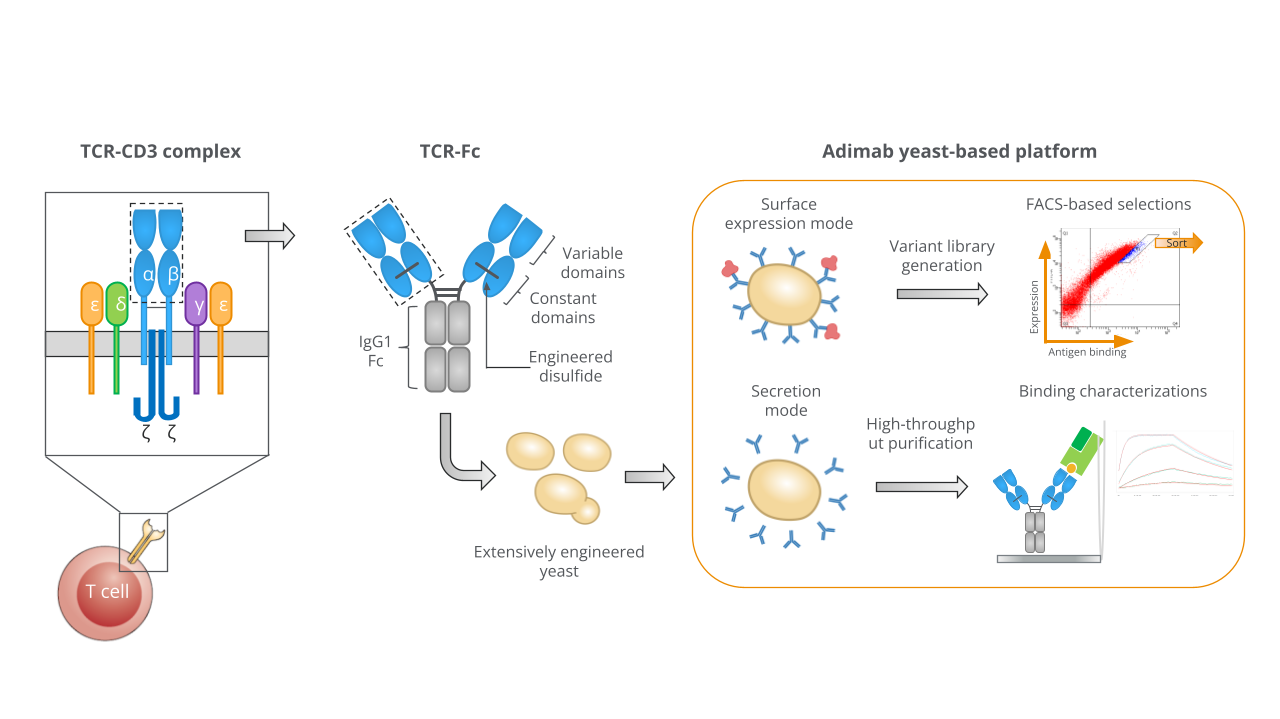
Yeast-based platform for TCR expression and engineering. A soluble TCR with engineered constant regions expressed in a bivalent IgG-like format.
Data-guided engineering of functional diversity
Our approach diversifies all six complementarity-determining regions (CDRs) across both TCR chains to explore structural and sequence space at the peptide and HLA interfaces. Deep mutational scanning (DMS) informs which mutations improve affinity while maintaining specificity, guiding recombination to generate optimized variants efficiently. The process combines quantitative binding data, flow cytometry–based selections, and biophysical validation to converge on molecules with sub-nanomolar affinities, high expression yields, and minimal mutational burden.
Results at a glance
Accelerated path to therapeutic leads
Our high-throughput workflow, from library design and selection to characterization, typically concludes within four to six months. This yields a set of stable, high-affinity, and highly specific TCRs ready to advance into therapeutic formats such as bispecifics.
This white paper provides detailed kinetic data, experimental workflows, and design strategies illustrating how our proprietary yeast-based system overcomes the traditional bottlenecks in TCR affinity engineering.